

§ 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. These tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19, under Section 564(b)(1) of the Act, 21 U.S.C. ID NOW COVID-19 and BinaxNOW COVID-19 Ag Card have been authorized by FDA under an EUA for use by authorized laboratories only for the detection of nucleic acid or proteins from SARS-CoV-2, and not for any other viruses or pathogens. Abbott was also named to Fortune's 2020 " Change the World" list for expanding access to quality primary care and diagnostic testing in Rwanda, and for advancing innovative testing to confront the COVID-19 pandemic.Abbott was recognized as Fast Company's " World Changing Company of the Year" for its Piccolo innovation, its work advancing the careers of underrepresented people in STEM, initiatives to fight malaria around the world and for the company's COVID-19 tests.It was also recognized in the R&D 100 Awards, which highlights the most prestigious innovations and revolutionary advancements in science and technology. Though smaller-than-a-pea, our Amplatzer Piccolo™ Occluder - which treats preemie and newborn babies with an opening in their hearts - received an Edison Award for its human-centered design in prenatal and infant care.MitraClip™ System, the world's leading minimally invasive mitral valve repair device, was honored by Prix Galien USA Award for Best Medical Technology.And our ID NOW™ COVID-19 rapid molecular test was recognized by ChicagoInno as a 2020 Inno on Fire winner, in addition to being named a Top Innovation of 2020 by The Scientist. BinaxNOW was also recognized as a top innovation by the Chicago Innovation Awards.Popular Science’s recognition comes on the heels of a number of recent honors for Abbott: Learn more about Abbott’s COVID-19 tests here. We’ve developed seven COVID-19 tests and have committed massive investments in these technologies, infrastructure and manufacturing to get our tests to as many people as we can. While we’re honored to have received this recognition from Popular Science, our job isn’t done.Īs leaders in infectious disease testing, teams of our scientists have worked around the clock to develop a variety of tests to help healthcare professionals tackle COVID-19, from large-scale lab testing to near-patient or point-of-care testing. This combined BinaxNOW and NAVICA solution is helping to better inform places where people gather in large numbers, such as schools and workplaces. This allows healthcare providers to test a lot of people a lot faster with a single-use test that requires no equipment.

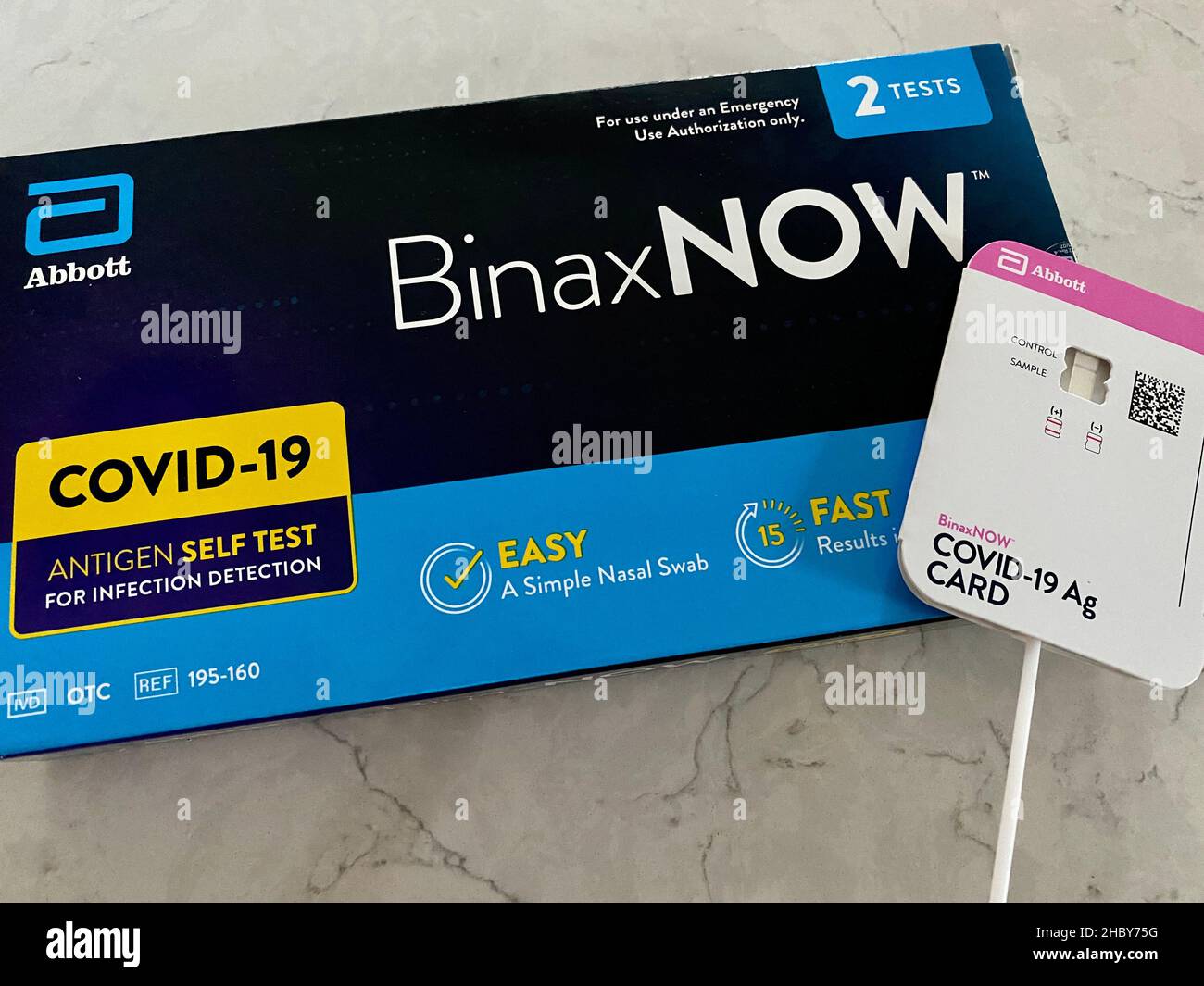
We designed BinaxNOW using proven lateral flow technology, similar to an at-home pregnancy test. With a rapid, reliable test result, people who test positive can take steps to ensure they isolate themselves to help prevent spread. And antigen tests, like BinaxNOW, are an important piece of the broader COVID-19 testing strategy.Īntigen tests detect whether a person has the virus, right now, and can serve as a first line of defense to help identify those people that are infected and contagious. In this critical moment in the pandemic, testing remains fundamental in our ability to help slow the spread of the disease.
#Binax now tests portable#
The BinaxNOW COVID-19 Ag Card - our fast, reliable, affordable and portable rapid test that can be used at mass scale - has been recognized as one of Popular Science’s “Best of What’s New” for 2020 Grand Award Winners.Īlong with the complementary NAVICA mobile app, they combine as one of the top 10 game-changing innovations in the magazine’s Health category.


 0 kommentar(er)
0 kommentar(er)
